Research
Nanocarbons, represented by graphene, carbon nanotubes, and fullerenes, have received much attention as next-generation functional materials. In addition, hetero-nanocarbons, in which specific carbons in nanocarbons are replaced by heteroatoms (boron, nitrogen, phosphorus, etc.), are predicted to have unique physical properties based on theoretical calculations. However, their synthesis is mainly based on chemical vapor deposition using small-molecule precursors or thermal annealing between nanocarbons and the precursors, which makes it difficult to control the position and number of heteroatoms introduced. As a result, most studies have not been beyond superficial research using mixtures. Therefore, we are conducting research with the goal of precise synthesis of heteronanocarbons and deepening the field as an academic discipline through the synthesis and applications in materials chemistry.
Development of tandem hetero-Friedel–Crafts reactions toward giant π-conjugated systems with heteroatoms
Heteroatom-containing π-conjugated compounds have been widely studied due to their excellent optical and electrochemical properties. However, lack of the suitable synthetic methods makes it difficult to introduce heteroatoms at the ring junctions. Therefore, we have established a novel methodology, namely “tandem hetero-Friedel–Crafts reaction”, to construct a polycyclic skeleton containing heteroatoms at the ring junctions in a one-pot manner. Moreover, by carrying out this reaction in a continuous intra- and intermolecular manner, one-shot synthesis of hetero-nanographenes and heterohelicenes with giant π-conjugated systems has been achieved. These are useful not only as novel optoelectronic materials but also as synthetic intermediates for hetero-nanocarbons.
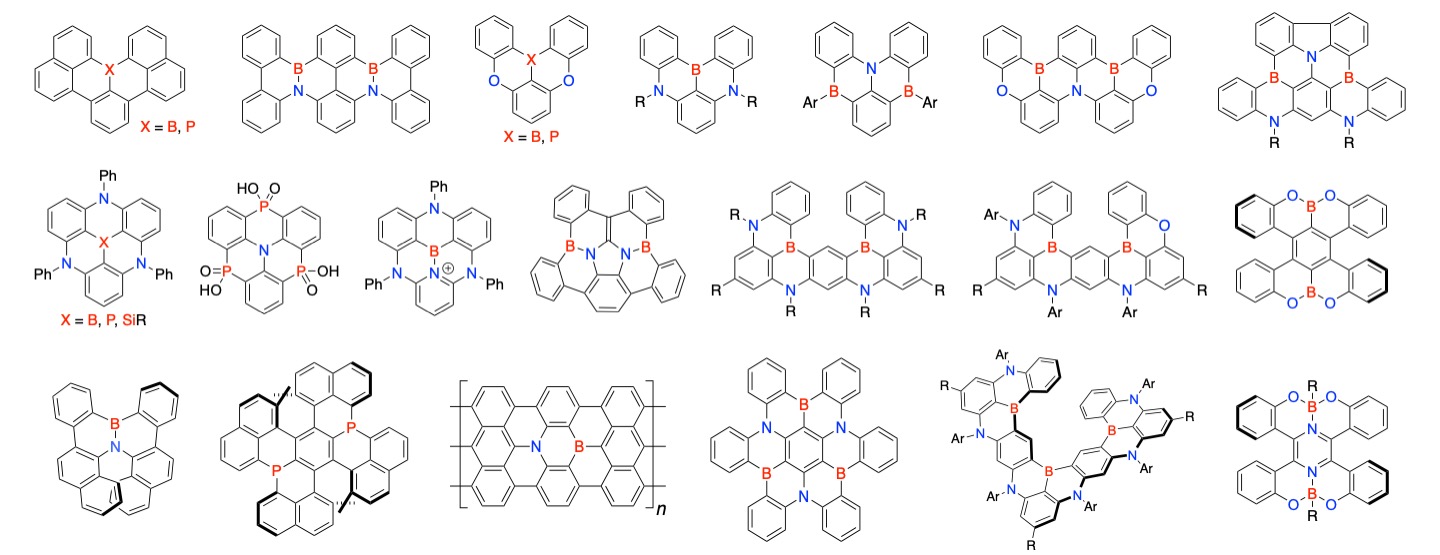
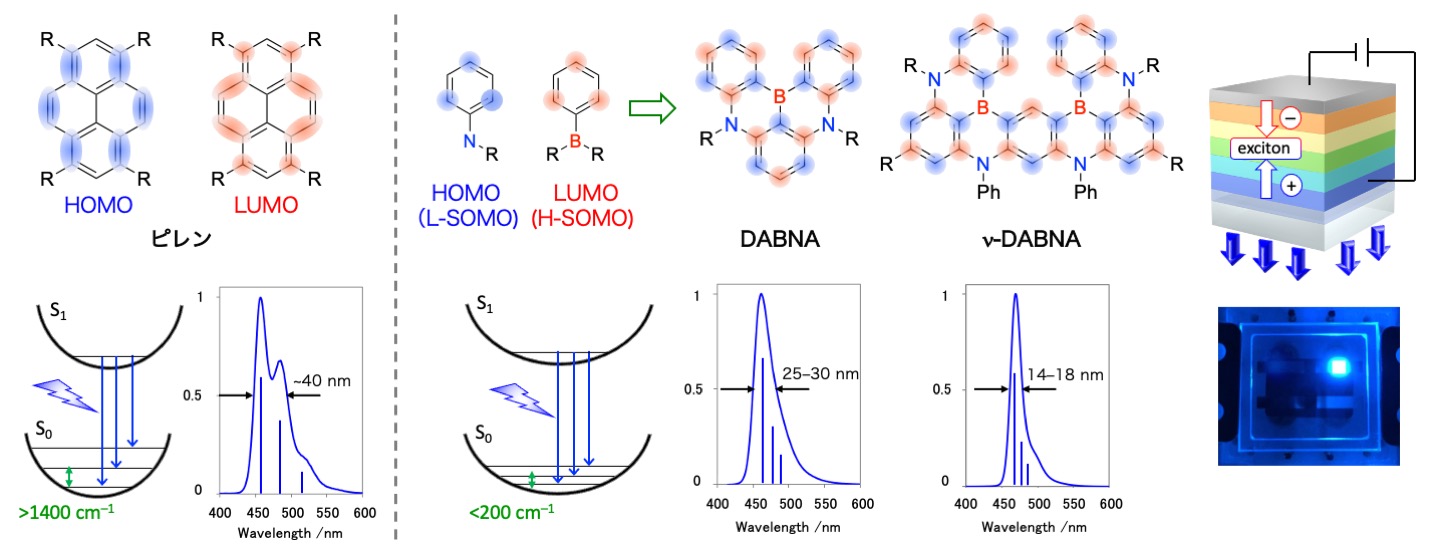
Development of narrow-band emitters based on multiple resonance effect
Since the frontier orbitals of organic light-emitting materials are mainly located between atoms, S1–S0 transitions are accompanied by bond stretching. As a result, the materials exhibit several emission peaks corresponding to electronic transitions from ground states of S1 to each vibrational states of S0, which causes broadening of emission spectra (i.e. vibronic spectra). To overcome this intrinsic problem, we introduced boron and nitrogen(or oxygen) atoms at 1,2-positions on the benzene ring and localized the HOMO and LUMO alternately on the adjacent carbons through their “multiple resonance effect”, thereby completely suppressing the vibronic coupling with the stretching vibration. Under this design principle, a wide variety of “DABNA” showing narrowband emission with a full width at half maximum of less than 30 nm with a fluorescence quantum yield of nearly 100% have been developed in academia and industry, and now widely utilized in commercial OLED displays. Furthermore, we have succeeded in developing “ν-DABNA,” which exhibits ultra-narrowband emission (a full width at half maximum of less than 20 nm) surpassing inorganic light-emitting materials. These materials have excellent thermal activation delayed fluorescence (TADF) properties, which can improve energy conversion efficiency by a factor of 2 to 3 compared to the commercial displays using DABNA derivatives.